Address:
- 20378 Seneca Meadows Pkwy,
- Germantown, MD 20876
Interleukin-4 is an additional anti-inflammatory cytokine that specifically suppresses T cell activation while also suppressing macrophage and monocyte inflammatory responses.
The scFv scaffold is used to enrich inflamed tissue with the anti-inflammatory cytokines targeting MAdCAM for Crohn’s and colitis, VEGFR2 for Psoriasis and Rheumatoid Arthritis, or CD14 for sepsis.
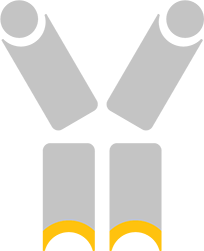
Interleukin-10 is a potent anti-inflammatory cytokine and the low affinity variant developed by Deka does not activate T cells at any dose
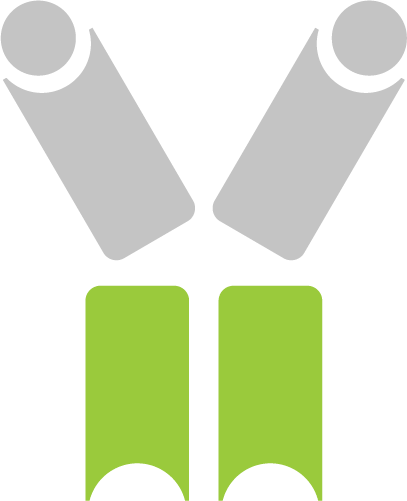
Each protein subunit of the Diakine™ (e.g. interleukin-4, interleukin-10, variable heavy and variable light chain) is linked in series as a single chain via simple and flexible protein linkers. Due to the orientation of the amino and carboxy termini of each protein, the Diakine™ exhibits a three- dimensional structure and stability similar to antibodies.
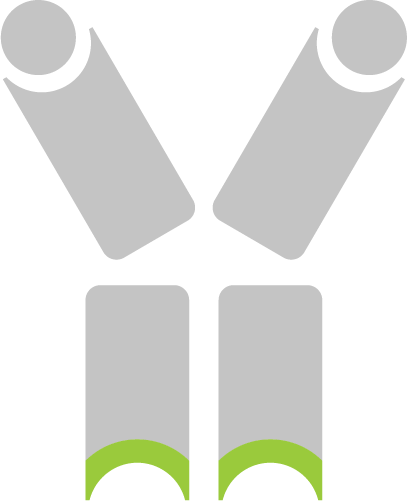
The complementarity determining regions (CDR’s) from other antibodies are grafted into the scFv scaffold sequence to confer specificity for targets expressed in inflamed tissue or on inflammatory cells (e.g., CD14, MAdCAM, VEGFR2 receptors).
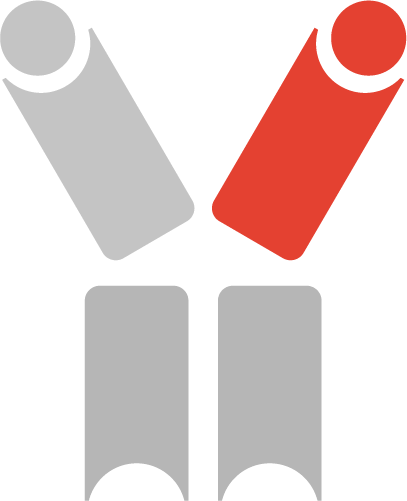
The complementarity determining regions (CDR’s) from other antibodies are grafted into the scFv scaffold sequence to confer specificity for tumor antigens (e.g., EGFR, HER2, VEGFR receptors).
Interleukin-10 is nature’s anti-inflammatory cytokine, which at certain doses can also activate anti-cancer T cells. By individually coupling the stimulatory cytokines with IL-10, each Diakine™ controls toxic inflammation, while potently activating the patient’s immune system to seek out and destroy tumors.
Each protein subunit of the Diakine™ (e.g. interleukin-2, interleukin-10, variable heavy and variable light chain) is linked in series as a single chain via simple and flexible protein linkers. Due to the orientation of the amino and carboxy termini of each protein, the Diakine™ exhibits a three- dimensional structure and stability similar to antibodies.
The single chain variable fragment (scFv) scaffold is used to enrich the DiakineTM in the tumor microenvironment. The scFv is comprised of a variable heavy and variable light chain derived from a human antibody.

Stimulatory cytokine (e.g., interleukin-2, interleukin-12, interferon alpha) are small proteins that can activate immune cells (e.g., T and NK cells) to selectively destroy cancer cells, but can also cause toxic inflammation.

Vice President, Technical Operations
Dave is a Biologics Operations leader specializing in the product lifecycle from initial process scale-up through commercial launch. His work experience includes operations leadership roles at Cytovance Biologics, Boehringer Ingelheim, and Catalent Cell & Gene Therapy. Throughout his career, Dave has had oversight and accountability for over 50 biologic and gene therapy clinical production campaigns, including 5 successful commercial launches. Dave is passionate about finding innovative scale-up solutions to improve access to life-saving biologics.

Biography
Dave is a Biologics Operations leader specializing in the product lifecycle from initial process scale-up through commercial launch. His work experience includes operations leadership roles at Cytovance Biologics, Boehringer Ingelheim, and Catalent Cell & Gene Therapy. Throughout his career, Dave has had oversight and accountability for over 50 biologic and gene therapy clinical production campaigns, including 5 successful commercial launches. Dave is passionate about finding innovative scale-up solutions to improve access to life-saving biologics.

Vice President, Clinical Operations
Deb has over 27 years working in oncology drug development, from bench research at the University of Wisconsin, to medical affairs and clinical development roles within pharma and CRO. She has held various leadership positions within small to large size pharma (US Bioscience, Eisai, MGI Pharma, MedImmune) as well as CROs (IQVIA, ICON, Synteract). She was most recently the Sr. Vice President of Oncology Strategy and Innovation at ErgoMed.
She brings a deep understanding of oncology and hemato-oncology clinical development landscape, along with established relationships with thought leaders and investigators globally.
Deb has an undergraduate degree in Pharmacology/Toxicology from the University of Wisconsin-Madison, and an MBA from the University of Wisconsin-Oshkosh.
She is also a cancer survivor, published author and patient advocate.

Biography
Deb has over 27 years working in oncology drug development, from bench research at the University of Wisconsin, to medical affairs and clinical development roles within pharma and CRO. She has held various leadership positions within small to large size pharma (US Bioscience, Eisai, MGI Pharma, MedImmune) as well as CROs (IQVIA, ICON, Synteract). She was most recently the Sr. Vice President of Oncology Strategy and Innovation at ErgoMed.
She brings a deep understanding of oncology and hemato-oncology clinical development landscape, along with established relationships with thought leaders and investigators globally.
Deb has an undergraduate degree in Pharmacology/Toxicology from the University of Wisconsin-Madison, and an MBA from the University of Wisconsin-Oshkosh.
She is also a cancer survivor, published author and patient advocate

General Counsel
Chris is a registered U.S. Patent attorney with over two decades of patent law experience. Before Deka, Chris practiced law at Arent Fox and Hunton & Williams helping life science, chemical, medical device, and agricultural clients with a variety of intellectual property issues. Chris also served as a primary patent examiner at the U.S. Patent & Trademark Office in the immunology-oncology arts. Chris also conducted angiogenesis research at the National Institutes of Health, National Institutes of Dental and Craniofacial Research and at Thomas Jefferson University, Cardeza Foundation for Hematologic Research. Chris is licensed to practice law in Washington, D.C and is admitted to the Supreme Court of the U.S. and the U.S. Court of Appeals, Federal Circuit.

Biography
Chris is a registered U.S. Patent attorney with over two decades of patent law experience. Before Deka, Chris practiced law at Arent Fox and Hunton & Williams helping life science, chemical, medical device, and agricultural clients with a variety of intellectual property issues. Chris also served as a primary patent examiner at the U.S. Patent & Trademark Office in the immunology-oncology arts. Chris also conducted angiogenesis research at the National Institutes of Health, National Institutes of Dental and Craniofacial Research and at Thomas Jefferson University, Cardeza Foundation for Hematologic Research. Chris is licensed to practice law in Washington, D.C and is admitted to the Supreme Court of the U.S. and the U.S. Court of Appeals, Federal Circuit.

Co-Founder, Chief Business Officer
Pavel is a strategic healthcare entrepreneur specializing in developing external collaborations focused on biotechnology, precision medicine, digital health, and diagnostics within the life sciences industry. His 18 years of experience has included roles at OriGene, Suburban Hospital, BioReliance by SAFC, and MedImmune/AstraZeneca.

Biography
Pavel is a strategic healthcare entrepreneur specializing in developing external collaborations focused on biotechnology, precision medicine, digital health, and diagnostics within the life sciences industry. His 18 years of experience has included roles at OriGene, Suburban Hospital, BioReliance by SAFC, and MedImmune/AstraZeneca.

Senior Clinical Advisor & Interim Chief Medical Officer

Biography

President & CEO
John has spent over 30 years researching and developing various technologies associated with IL-10. His work experience included various leadership roles at Anergen, Introgen Therapeutics, Bacterial BarCodes, Schering-Plough, Merck, Targenics, ARMO BioSciences, ACIR BioSciences, and MedImmune/AstraZeneca. John’s primary focus has been on discovery research at the interface between cancer biology and the adaptive immune response.

Biography
John has spent over 30 years researching and developing various technologies associated with IL-10. His work experience included various leadership roles at Anergen, Introgen Therapeutics, Bacterial BarCodes, Schering-Plough, Merck, Targenics, ARMO BioSciences, ACIR BioSciences, and MedImmune /AstraZeneca. John’s primary focus has been on discovery research at the interface between cancer biology and the adaptive immune response.